Ka & Tm
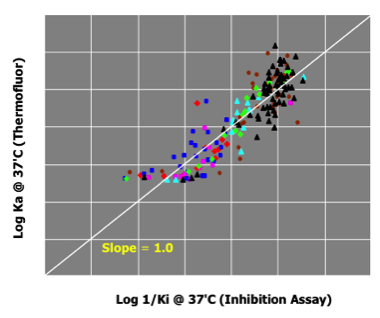
Thrombin Inhibitors
The magnitude of DeltaTm associated with ligand binding depends on the thermodynamics of protein stability, particularly the enthalpy of protein unfolding.
A protein's enthalpy of unfolding can be directly measured using DSC.
Although the enthalpy of protein folding can differ for each individual protein, multiple ligands tested against the same protein will show DeltaTm effects that are proportional to Ka.
For a detailed treatment, based on inhibitors of carbonic anhydrase, see references below.
Thermodynamic stability of carbonic anhydrase: measurements of binding affinity and stoichiometry using ThermoFluor. Matulis D,et.al. Biochemistry. 2005 Apr 5;44(13):5258-66. [Enzymology application with detailed mathematical analysis of thermofluor binding experiments] PMID: 15794662
PDF
Inhibition of carbonic anhydrase-II by sulfamate and sulfamide groups: an investigation involving direct thermodynamic binding measurements. Klinger AL,et.al. J Med Chem. 2006 Jun 15;49(12):3496-500. [Enzymology application with detailed mathematical analysis of thermofluor binding experiments] PMID: 16759092
Carbonic anhydrase-II inhibition. what are the true enzyme-inhibitory properties of the sulfamide cognate of topiramate? Maryanoff, BE, et. al. 2008 Apr 24;51(8):2518-21 [Thermoflour analysisi of inhibitor binding.] PMID: 18363349