Publications
My PhD thesis work at UCSD involved the structure determination of cytochrome c2 from a photosynthetic bacterium. The protein was being produced and biochemically studied in Martin Kamen's lab at UCSD. Comparative structural studies of cytochromes and accompanying biochemical experiments demonstrated the importance of electrostatic interactions in facilitating electron transport between freely diffusing electron transfer proteins. I continued working on the structure determination of electron transfer proteins when I joined the faculty at the University of Arizona, where I collaborated with Mike Cusanovich's lab. I also carried out modeling studies of electron-transfer protein complexes, which were among the first molecular modeling studies of protein-protein interactions. Later, when computational methods emerged that allowed visualization of electrostatic fields around proteins, these methods were applied to numerous systems including electron transfer and other interacting systems where electrostatics were known to have functional importance.
1. Enzymic Redox Reactions of Cytochrome c.
K.A. Davis, Y. Hatefi, F.R. Salemme, and M.D. Kamen Biochem. Biophys. Res. Commun. 1972; 49: 1329-1335
K.A. Davis, Y. Hatefi, F.R. Salemme, and M.D. Kamen Biochem. Biophys. Res. Commun. 1972; 49: 1329-1335
7. Structural Bases for Function in Cytochrome c: An Interpretation of Comparative X-ray and Biochemical Data, F.R. Salemme, J. Kraut, and M.D. Kamen, J. Biol. Chem. 1973; 248: 7701-7716.
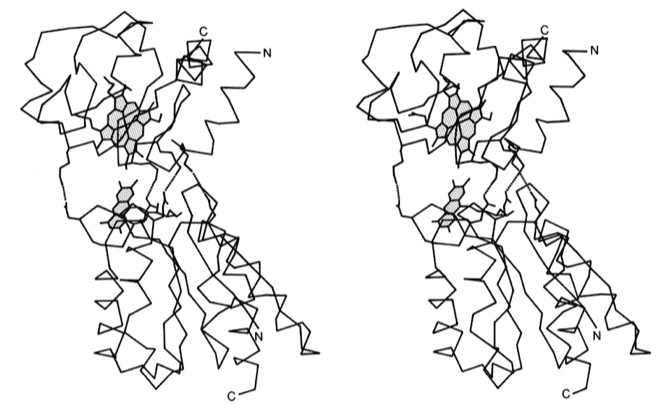
9. A Hypothetical Structure for an Intermolecular Electron Transfer Complex of Cytochromes c and b5, F.R. Salemme, J. Mol. Biol. 1976; 102: 563-568.
A basic question about electron transfer reactions between proteins centered on whether electron transfer occurred through direct contact between orbitals of metal-containing prosthetic groups, or through intermediate (presumably aromatic) amino acid side chains. Cytochromes C and b5 were known to react in an ionic strength dependent manner, suggesting complementary charge pairing between the proteins to facilitate electron transfer. Since this study was carried out before computer graphics, physical models were built to examine the proteins' complementary surface features, which were then used to create a least squares docked complex. In the event, it turns out that the heme prosthetic groups cannot physically contact each other in the complex, suggesting that ET takes place through a short-range tunneling process. This model is among the first protein protein complexes modeled using X-ray structures.
10. Structure and Function of Cytochrome c, F.R. Salemme, Annual Review of Biochemistry 1977; 46: 229-329.
This review article summarized and compared the common features of prokaryotic and eukaryotic Cytochromes c. These generally included a common structural fold, common heme iron ligands and covalent mode of heme attachment, an invariant PHE residue adjacent the heme where it was surface-exposed, and a ring of positively charged LYS residues at the surface that allowed electrostatic orientation of the cytochrome molecule to facilitate electron transfer reaction.
14. Protein Dynamics, Potential Regulation and Redox Coupled Conformational Changes in Cytochrome c.
F.R. Salemme Frontiers of Biological Energetics (P.L. Dutton, S. Leigh, A. Scarpa, eds.). Academic Press 1978; 83-89
F.R. Salemme Frontiers of Biological Energetics (P.L. Dutton, S. Leigh, A. Scarpa, eds.). Academic Press 1978; 83-89
15. Structure-Function Relationships in Biological Electron Transport Proteins.
F. R. Salemme Tunneling in Biological Systems, (B. Chance, ed.) Academic Press 1979; 523-541
F. R. Salemme Tunneling in Biological Systems, (B. Chance, ed.) Academic Press 1979; 523-541
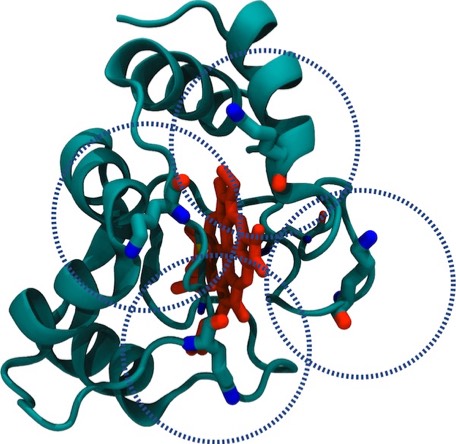
30. Transient Kinetics of Electron Transfer Reactions of Flavodoxin: Ionic Strength Dependence of Semiquinone Oxidation by Cytochrome c, Ferricyanide, and Ferri EDTA and Computer Modelling of Reaction Complexes, R.P. Simondsen, P.C. Weber, F.R. Salemme, and G. Tollin, Biochemistry 1983; 21: 6366-6375.
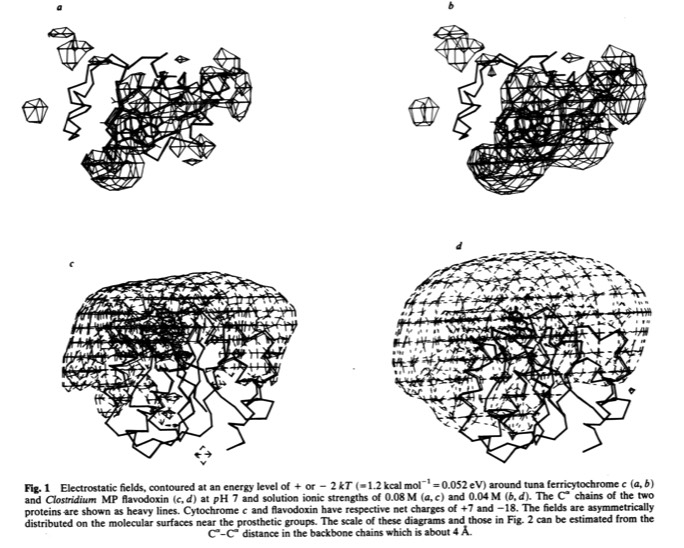
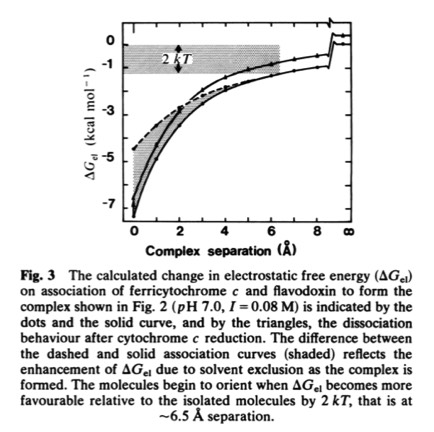
33. Electrostatic Orientation During Electron Transfer Between Flavodoxin and Cytochrome c, J.B. Matthew, P.C. Weber, F.R. Salemme, and F.M. Richards, Nature 1983; 301: 169-171
This paper with Jim Matthew, carried out while on sabbatical at Yale, was among the first to illustrate the long range electrostatic field effects that could arise from localized charge distributions on protein surfaces. Such field effects are important for many types of restricted diffusion phenomena in biology - including for example 2D diffusion of electron transport proteins on membranes and 1D diffusion of DNA binding proteins along DNA.