Publications
Following undergraduate work with Fred Richards on the structure determination of Ribonuclease-S, I decided to pursue a PhD with Joe Kraut at UCSD in protein crystallography. I developed an interest in redox protein structure for my PhD thesis and continued studies on redox proteins during my academic career at UAZ. Subsequently, I have used protein crystallography as a means of investigating a number of questions in structural biology and drug discovery, ranging over the majority of my academic and professional career.
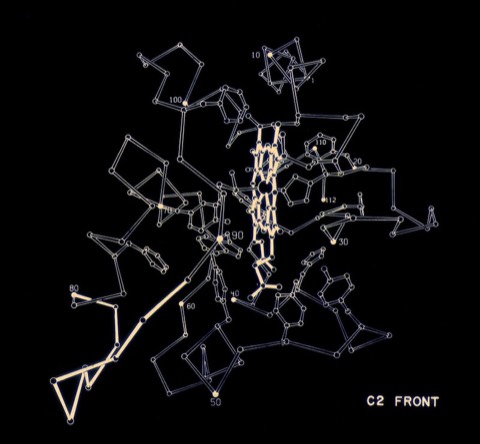
5. Atomic Coordinates for Ferricytochrome c2 of Rhodospirillum rubrum.
F.R. Salemme, S.T. Freer, R.A. Alden, and J. Kraut Biochem. Biophys. Res. Commun 1973; 54: 47-51
F.R. Salemme, S.T. Freer, R.A. Alden, and J. Kraut Biochem. Biophys. Res. Commun 1973; 54: 47-51
6. The Structure of Oxidized Cytochrome c2 of Rhodospirillum rubrum.
F. R. Salemme, S.T. Freer, R.A. Alden, Ng.H. Xuong, and J. Kraut. J. Biol. Chem. 1973; 248: 3910-3921.
F. R. Salemme, S.T. Freer, R.A. Alden, Ng.H. Xuong, and J. Kraut. J. Biol. Chem. 1973; 248: 3910-3921.
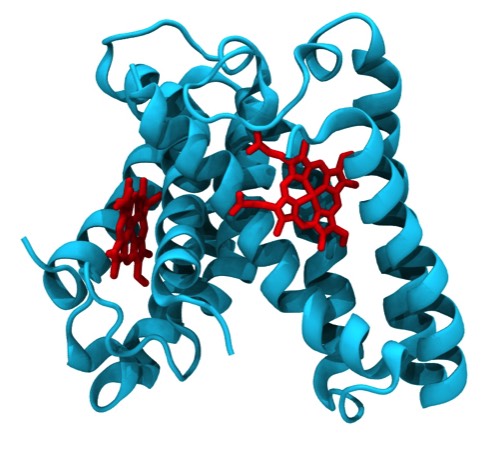
While a graduate student at UCSD in Joe Kraut's lab, I formed a relationship with Martin Kamen who was working on the cytochromes of photosynthetic bacteria. Cytochrome c2 from R. rubrum showed homology with mammalian cytochrome c, but at that time it was unclear if the proteins were structurally related as well. In the event, it was found that the R. rubrum structure was quite similar to the horse cytochrome structure done by Dick Dickerson, then at CalTech.
8. Preliminary Crystallographic Data for Cytochrome c' of Rhodopseudomonas palustris.
F.R. Salemme Arch. Biochem. Biophys. 1974; 163: 423-425
F.R. Salemme Arch. Biochem. Biophys. 1974; 163: 423-425
11. The Structure of Chlorobium thiosulfatophilum cytochrome c555: A Primitive Low-potential c-type Cytochrome.
Z.R. Korszun and F.R. Salemme Proc. Natl. Acad. Sci. USA 1977; 74: 5244-5247
Z.R. Korszun and F.R. Salemme Proc. Natl. Acad. Sci. USA 1977; 74: 5244-5247
12. Preliminary Crystallographic Data for Cytochromes c' of Rhodopseudomonas capsulata and Rhodospirillum molischianum.
P.C. Weber and F.R. Salemme J.
P.C. Weber and F.R. Salemme J.
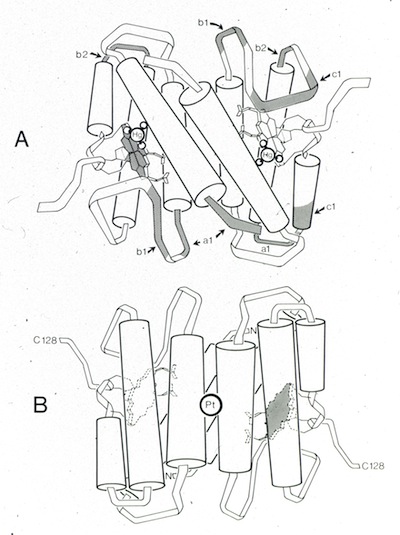
19. Structure of Cytochrome c': A Dimeric High-Spin Haem Protein.
P. C. Weber, R.G. Bartsch, M.A. Cusanovich, R.C. Hamlin, A. Howard, S.R. Jordan, M.D. Kamen, T.E. Meyer, D.W. Weatherford, Ng.H. Xuong, and F.R. Salemme, Nature 1980; 286: 302-304.
P. C. Weber, R.G. Bartsch, M.A. Cusanovich, R.C. Hamlin, A. Howard, S.R. Jordan, M.D. Kamen, T.E. Meyer, D.W. Weatherford, Ng.H. Xuong, and F.R. Salemme, Nature 1980; 286: 302-304.
Following a move to the University of Arizona, I continued working of microbial cytochrome structures being studied in Mike Cusanovich's lab. Cytochrome c' turned out to be a prototypical 4-alpha-helical bundle protein. Numerous cytochrome c' structures have subsequently been determined using X-ray crystallography, although the specific function of the protein remains unknown.
20. Crystallographic Structure of Rhodospirillum molischianum Ferricytochrome c' at 2.5 Å Resolution.
P.C. Weber, A. Howard, Ng.H. Xuong, and F.R. Salemme J. Mol. Biol. 1981; 153: 399-425
P.C. Weber, A. Howard, Ng.H. Xuong, and F.R. Salemme J. Mol. Biol. 1981; 153: 399-425
25. Cytochrome c’: A Dimeric High-Spin Heme Protein.
P.C. Weber and F.R. Salemme Symposium on Interaction Between Iron and Proteins in Oxygen and Electron Transport, C. Ho., ed., Elsevier North Holland, Inc. 1982; 57-59
P.C. Weber and F.R. Salemme Symposium on Interaction Between Iron and Proteins in Oxygen and Electron Transport, C. Ho., ed., Elsevier North Holland, Inc. 1982; 57-59
28. Preliminary Crystallographic Data for Chromatium Flavocytochrome c552.
F.R. Salemme J. Mol. Biol. 1982; 159: 551-552
F.R. Salemme J. Mol. Biol. 1982; 159: 551-552
39. The 2Å Resolution Structure of a Thermostable Ribonuclease-A Chemically Cross-linked Between Lysine residues 7 and 41.
P.C.Weber, S. Sheriff, D.H. Ohlendorf, B.C. Finzel, and F.R. Salemme, Proc. Natl. Acad. Sci. USA 1985; 82: 8473-8477.
P.C.Weber, S. Sheriff, D.H. Ohlendorf, B.C. Finzel, and F.R. Salemme, Proc. Natl. Acad. Sci. USA 1985; 82: 8473-8477.
This work was the result of a collaboration with Harold Scheraga. Harold had demonstrated that ribonuclease incorporating an additional chemical cross-link was stabilized, an effect attributed to an entropy decrease in partially unfolded states of the protein. The structure determination demonstrated that the folded state of the chemically modified protein was essentially identical to the native protein structure.
40. The Structure of Ferricytochrome c' from Rhodospirillum molischianum at 1.67 Å Resolution, B.C. Finzel, P.C. Weber, K.D. Hardman, and F.R. Salemme, J. Mol. Biol. 1985; 186: 627-643.
42. Preliminary Crystallographic Data for Cross-linked (Lysine 7-Lysine 41) -Ribonuclease A.
P.C. Weber, F.R.Salemme, S.H. Lin, Y. Konishi, and H.A. Scheraga J. Mol. Biol. 1985; 181: 453
P.C. Weber, F.R.Salemme, S.H. Lin, Y. Konishi, and H.A. Scheraga J. Mol. Biol. 1985; 181: 453
46. Preliminary Crystallographic Data for Streptomyces avidinii Streptavidin
P.C. Weber, M.J.Cox, F.R.Salemme, and D.H. Ohlendorf J. Biol. Chem. 1987; 262: 12728-9
P.C. Weber, M.J.Cox, F.R.Salemme, and D.H. Ohlendorf J. Biol. Chem. 1987; 262: 12728-9
53. Structural Origins of High Affinity Biotin Binding to Streptavidin.
P.C. Weber, D.H. Ohlendorf, J.J. Wendoloski, F.R. Salemme, Science 1989; 243: 85-88
P.C. Weber, D.H. Ohlendorf, J.J. Wendoloski, F.R. Salemme, Science 1989; 243: 85-88
An answer to the long standing question regarding the high affinity of avidin and streptavidin for the small molecule ligand biotin (More here).
56. X-Ray Structural Studies of the Cytokine Interleukin 1-ß.
A.C. Treharne, D.H.Ohlendorf, P.C.Weber, J.J. Wendoloski, F.R. Salemme Cytokines and Lipocortins in Inflammation and Differentiation, (M. Melli and Luca Parente, eds.) Wiley Liss and Inc. N.Y. N.Y. 1990; 309 -320.
A.C. Treharne, D.H.Ohlendorf, P.C.Weber, J.J. Wendoloski, F.R. Salemme Cytokines and Lipocortins in Inflammation and Differentiation, (M. Melli and Luca Parente, eds.) Wiley Liss and Inc. N.Y. N.Y. 1990; 309 -320.
58. Crystal Structure of a Retroviral Protease from Avian Myeloblastosis Associated Virus.
S.I. Foundling, F.R. Salemme, B. Korant, J.J. Wendoloski, P.C. Weber, A.C. Treharne, M. C. Schadt, M. Jaskolski, M. Miller, A. Wlodawer, P. Strop, V. Kostka, J. Sedlacek, D.H. Ohlendorf Current Communications in Molecular Biology: Viral Proteinases as Targets for Chemotherapy ( H.G. Krausslich, S. Oroszlan, E. Wimmer, eds.) 1990; 181-190.
S.I. Foundling, F.R. Salemme, B. Korant, J.J. Wendoloski, P.C. Weber, A.C. Treharne, M. C. Schadt, M. Jaskolski, M. Miller, A. Wlodawer, P. Strop, V. Kostka, J. Sedlacek, D.H. Ohlendorf Current Communications in Molecular Biology: Viral Proteinases as Targets for Chemotherapy ( H.G. Krausslich, S. Oroszlan, E. Wimmer, eds.) 1990; 181-190.
63. The Refined Crystal Structure of Bovine Pro-Phospholipase A2 at 1.6 Å Resolution.
B. C. Finzel, P.C. Weber, D.H. Ohlendorf, J.J. Wendoloski and F.R. Salemme Acta Cryst. 1991; B47: 814-816.
B. C. Finzel, P.C. Weber, D.H. Ohlendorf, J.J. Wendoloski and F.R. Salemme Acta Cryst. 1991; B47: 814-816.
64. An Independent Crystallographic Refinement of Porcine Phospholipase A2 at 2.4 Å Resolution.
B.C. Finzel, D.H. Ohlendorf, P.C. Weber, and F.R. Salemme, Acta. Cryst. 1991; B47: 558-559.
B.C. Finzel, D.H. Ohlendorf, P.C. Weber, and F.R. Salemme, Acta. Cryst. 1991; B47: 558-559.
74. Structural Studies of the Retroviral Proteinase from Avian Myeloblastosis Associated Virus.
D.H. Ohlendorf, S.I. Foundling, J.J. Wendoloski, G. Sedlacek, P. Strop, and F.R. Salemme Proteins: Structure, Function,Genetics 1992; 14: 382-392.
D.H. Ohlendorf, S.I. Foundling, J.J. Wendoloski, G. Sedlacek, P. Strop, and F.R. Salemme Proteins: Structure, Function,Genetics 1992; 14: 382-392.