Precedents
Streptavidin Structure
Streptavidin
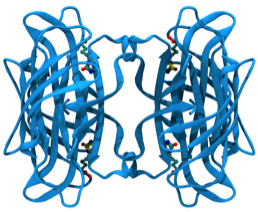
Imiplex founders determined the 3-dimensional structure of streptavidin and defined the physical origins of the high affinity binding of biotin in 1989.
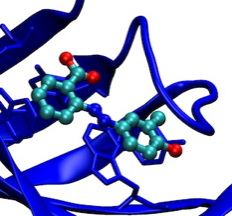
Streptavidin Ligand Design
Ligand Design
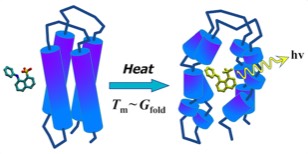
Structure-based design work on streptavidin ligands in 1991 resulted in the development of novel dye ligands useful for monitoring streptavidin-mediated assembly processes and additionally characterized the thermodynamics of ligand binding to streptavidin.

Ras P21 Reconstruction
Protein Structural Heuristics
Imiplex team members were among the first to implement computational methods for the automated construction of proteins from approximate chain traces using fragment superposition (FRAGLE) and context-dependent rotamer statistics (PROBIT) as recurrent structural motifs observed in known protein crystal structures. Modern extensions of these approaches, coupled both with advances in computational power and the explosion in known 3D crystal structures, had made such heuristic methods extremely efficient and effective in the design of new, stably folded protein motifs.
Protein Structural Stability
Maintaining or enhancing the structural stability of engineered proteins is key to insure both economic production and compatibility with the broadest range of process and product applications. Imiplex founders developed and commercialized thermofluor (aka thermal shift assay, differential scanning fluorimetry), a widely-used miniaturized, high-throughput method for the quantitative measurement protein structural stability.
Molecular Electronics
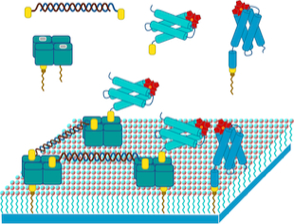
In 1992 Sligar and Salemme published an early speculative study outlining how streptavidin-linked structures assembled on lipid monolayers might provide the basis for molecular electronics devices.
NIST Technology Innovation Program Whitepaper:
Imiplex developed a whitepaper for the NIST Technology Innovation Program entitled “Scalable Architecture for Nanotechnology” outlining key considerations and advantages of protein-based nanotechnology.
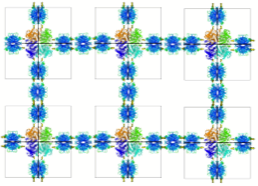
Ringler & Schulz 2003
Streptavidin-Linked 2-Dimensional Lattices
In 2003, Ringler and Schulz published a pioneering paper showing the ability to form 2D protein lattices linked by streptavidin on self-assembling monolayers, using a C4-symmetric aldolase multimer that had been modified to introduce reactive cysteine sites that could be chemically modified to attach biotin groups.