Redpoint Bio: Adventures in Taste (2004-2011)
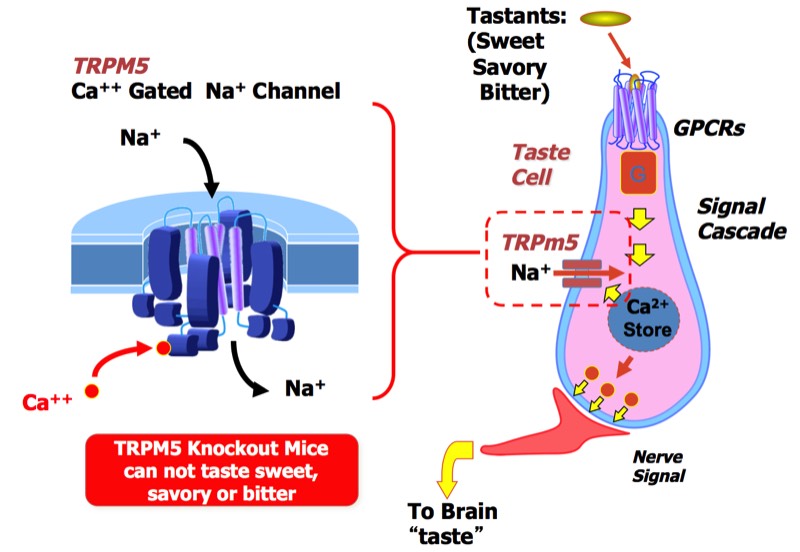
TRPM5 mechanism in taste. TRPM5 is a calcium-gated sodium channel that triggers downstream signaling when tastant molecules bind to specific GPCR receptors located on the taste cell surface. TRPM5 knockout animals were observed to be unable to sense sweet, bitter, and umami tastants, inferring a key role in the taste signal transduction system in animals.
Humans are known to have multiple bitter GPCR taste receptors, presumably evolved to facilitate avoidance of toxic materials in the environment. It is unknown whether a given bitter compound acts at a single receptor or across multiple receptors simultaneously. Many common drugs are very bitter, creating formulation difficulties for pediatric applications. One of Redpoint’s objectives was to find a TRPM5 blocker that could act as a universal bitter blocker for pediatric formulation applications. Unfortunately, it turns out that in addition to GPCRs, there are evidently additional mechanisms that contribute to aversive taste sensation. Making palatable pediatric formulations of many common medicines consequently remains a challenge.
Redpoint developed the MOG, and operant conditioning platform that allowed rats to perform taste discrimination assays in a high-throughput format.
US Patent 8,820,265
US Patent 10,052,058
US Patent 8,820,265
US Patent 10,052,058
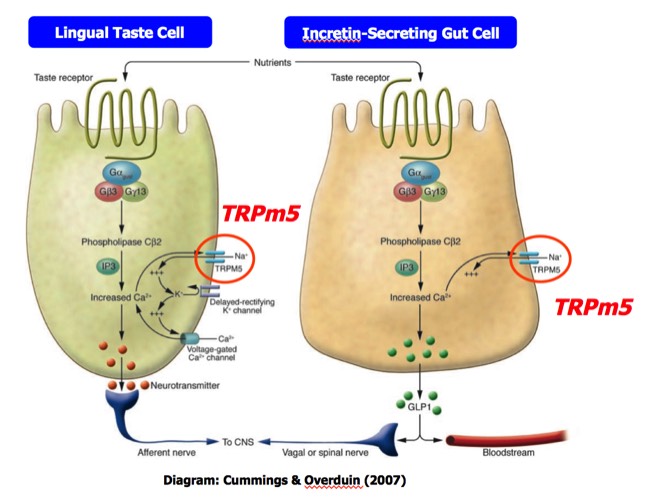
Several groups proposed that in addition to taste cells, the GPCR-TRPM5 signal transduction chain was also found in gut cells responsible for secretion of incretins like GLP-1, an important regulator of insulin secretion. This motivated Redpoint to repurpose small molecule modulators of TRPM5 originally developed for taste applications as potential diabetes drugs.
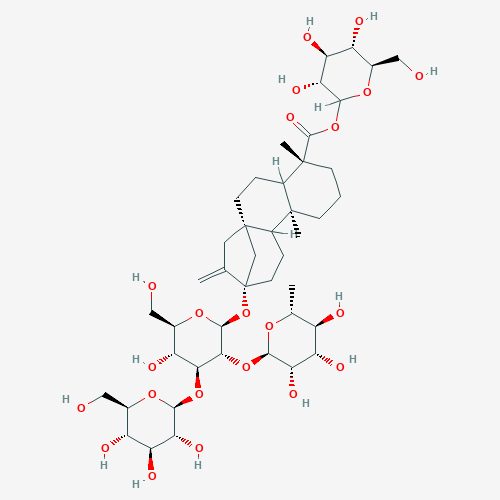
Rebaudioside C (RebC). This complex molecule is one of several steviol glycosides derived from stevia. Redpoint obtained GRAS certification for RebC for sweet taste enhancement applications in foods and beverages.
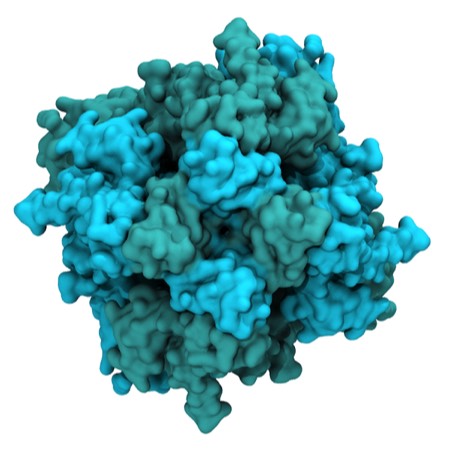
The crystal structure of TRPM4, a relative of TRPM5. The structure is a tetramer with the central ion-conducting channel located at the center of the four subunits as shown in the illustration. Crystal structures would have been very useful for the optimization of TRPM5 modulators, but were unavailable during the period when the discovery work was active.
Following the sale of 3DP in 2003, I elected not to stay on to assume a new role within J&J and so was assigned to the bench for 6 months while serving as an interim consultant for a nanotechnology company in Maryland. Meanwhile, my founding CFO at 3DP, Scott Horvitz, had gone on to organize a Series A venture financing for Linguagen, a local startup founded by Bob Margolskee (then at Mt Sinai School of Medicine in NYC) that was aimed at developing new taste modifying compounds using modern tools of molecular biology and drug discovery. Basically the company was following the lead of a West Coast biotech Senomyx which had developed a high throughput screening platform based on the newly-discovered sweet, umami, and bitter GPCR receptors.
The Linguagen investor syndicate included SROne and NJ Technology Ventures, as well as some more unconventional venture investment groups from Cargill, DuPont, and Danisco. After meeting with the investors, I agreed to join the company as CEO.
Although the company had been operating for some time, research was still in the early stages and the aggressive posture taken by Senomyx around GPCR taste receptor IP made strategy development a challenging prospect. Fortunately we were able to recruit some top talent, including Rusty Bryant and Kyle Palmer from Schering Plough and Phil Stein from BMS, to build the discovery infrastructure and define company strategy going forward.
As our discovery target, we decided to focus efforts on a calcium-gated sodium ion channel called TRPM5 which acted downstream from the tastant GPCRs in the taste cells of the tongue, and for which we had a reasonable IP position owing to work in the Margolskee lab. We established a full research capability, including channel molecular and cell biology, transgenic animals, screening HTS, cheminformatics and medicinal chemistry. Our idea was to find both agonists and antagonists of TRPM5 that could modulate taste downstream of the tastant GPCR receptors. We made initial progress rapidly and were able to cut a major multi-year discovery deal with Givaudan to both support the continuing research and license compounds that we discovered.
The Givaudan deal impacted our discovery program in ways that were initially unanticipated. For one, or cheminformatics work clearly indicated that that chemical compounds developed as drugs were systematically different from the compounds typically used in the food and beverage industries. Although partly an accident of history, novel chemical properties were potentially viewed as liabilities in achieving GRAS designation (Generally Regarded As Safe – a designation involving an approval process considerably less rigorous than an FDA drug approval) or end user acceptance. Since the TRPM5 modulators that we had discovered were derived from drug-like compound libraries, there was considerable pressure from Givaudan to find alternative sources of compounds to screen, preferably from natural product sources. Meanwhile, recognizing that there could potentially be other uses for our compounds beyond tastant modulation, we changed the name of the company from Linguagen to Redpoint Bio to reflect a broader focus on pharmaceutical applications.
Attitudes in the food and beverage industries were changing rapidly as the Givaudan collaboration progressed. This change was catalyzed by the introduction of the all-natural sweetener Truvia. Truvia is a low calorie sweetener developed through a collaboration between Coca-Cola and Cargill. Coca Cola had been investigating stevia glycosides as potential natural non-caloric sweeteners and had identified and obtained GRAS certification for a purified stevia leaf component rebaudioside-A (RebA). RebA initially tastes sweet but has a cloying bitter aftertaste. However, by combining RebA with erythritol, a non-metabolized and sweet poly-alcohol developed by Cargill, the bitter aftertaste of RebA can be suppressed, producing an acceptable product in Truvia. The success of Truvia excited the search for additional novel all-natural tastants and enhancers.
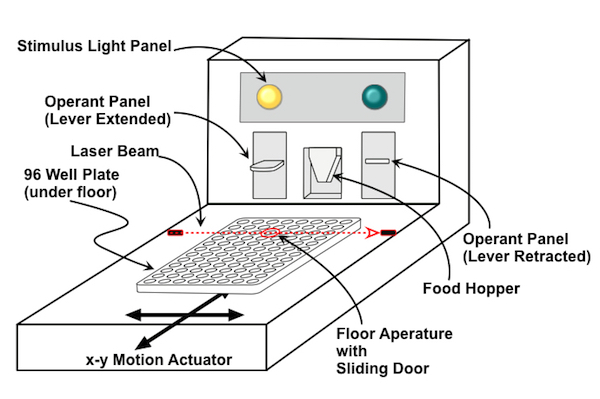
The desire to screen natural compounds to discover new all-natural tastants created a host of difficulties when applied to the cell-based assays we were using to identify TRPM5 modulators. Consequently Redpoint developed a totally new technology termed the MOG (Multiple Operant Gustometer) that relied on trained rats to make taste discriminations.
Although the operant taste discrimination assay (aka lickometer) had a long history in taste research, the MOG randomly presented samples from a 96-well micro-titer plate and dramatically increased throughput while producing manifestly better data than obtained using conventional methodology. MOG development was quarterbacked by Kyle Palmer who had an unusual background that encompassed both molecular pharmacology and behavioral sciences. We encountered some resistance from the beverage industry regarding the MOG approach both for its use of (very well treated) animals and because it was known the rats did not detect aspartame (a well-known synthetic sweetener) as sweet. Despite the skepticism, Redpoint was able to establish the effectiveness of rebaudioside-C (aka RebC – another stevia leaf component) as a sweetness enhancer using the MOG technology. Redpoint was able to gain GRAS certification for RebC and entered a development deal for commercialization with International Flavor and Fragrances (IFF) based on its discovery.
As a result of the paradigm shift to focus on natural products and a shift in Givaudan internal program strategy, Givaudan excercised an option to early terminate the TRPM5 program with Redpoint. Termination of the Givaudan collaboration raised several issues for the company, not the least among stockholders who had recently invested in the newly public company through a reverse merger PIPE (Private Investment in a Public Entity) transaction based on potential returns in the taste enhancement business.
At the same time that we were developing technology to find natural product tastants, we were initiating a shift in company direction to capitalize on the TRPM5 small-molecule program. This effort was responding to publications emanating from the Margolskee and other labs that, in addition to taste cells, TRPM5 had been found in gut and pancreatic islet cells, where it was inferred to participate in signal transduction chains involved in glucose-dependent insulin secretion. Consequently, it was expected that modulators of the channel could potentially act as glucose-dependent enhancers of insulin secretion, which would constitute a novel and highly valuable therapeutic for the treatment of diabetes.
We expanded our scientific team and began to evaluate both our TRPM5 enhancers and inhibitors for their efficacy in a number of cellular and animal models for insulin secretion.
We were excited to find that in both cellular assays and animal models that our TRPM5 inhibitors appeared to act as glucose-dependent insulin secretogogues. The only problem was that recently published work from the Margolskee lab describing the phenotype of a TRPM5 knockout mouse suggested that enhancers of the TRPM5 channel (used in our experiments as controls) should have secretogogue activity, not inhibitors
This apparent contradiction seriously impeded our ongoing deal activity around formation of a collaborative discovery program with a major pharma company, and ultimatel necessitated a major reduction in force to conserve cash.
In the event, it turns out that constitutive knockouts in metabolic pathway components are known to occasionally produce anomalous and unexpected phenotypes; the SUR1 KATPase that is the target of the glitazones being one relevant example.
In the event, we ultimately had to revert to out-licensing Redpoint’s research and technology assets in order to wind down the company.
On a final upbeat point, Kyle Palmer was able to evolve the MOG technology used for high-throughput rodent tastant discrimination into a totally novel operant system usable by humans. This has subsequently been developed as a key technology platform for the company Opertech Bio.
The Linguagen investor syndicate included SROne and NJ Technology Ventures, as well as some more unconventional venture investment groups from Cargill, DuPont, and Danisco. After meeting with the investors, I agreed to join the company as CEO.
Although the company had been operating for some time, research was still in the early stages and the aggressive posture taken by Senomyx around GPCR taste receptor IP made strategy development a challenging prospect. Fortunately we were able to recruit some top talent, including Rusty Bryant and Kyle Palmer from Schering Plough and Phil Stein from BMS, to build the discovery infrastructure and define company strategy going forward.
As our discovery target, we decided to focus efforts on a calcium-gated sodium ion channel called TRPM5 which acted downstream from the tastant GPCRs in the taste cells of the tongue, and for which we had a reasonable IP position owing to work in the Margolskee lab. We established a full research capability, including channel molecular and cell biology, transgenic animals, screening HTS, cheminformatics and medicinal chemistry. Our idea was to find both agonists and antagonists of TRPM5 that could modulate taste downstream of the tastant GPCR receptors. We made initial progress rapidly and were able to cut a major multi-year discovery deal with Givaudan to both support the continuing research and license compounds that we discovered.
The Givaudan deal impacted our discovery program in ways that were initially unanticipated. For one, or cheminformatics work clearly indicated that that chemical compounds developed as drugs were systematically different from the compounds typically used in the food and beverage industries. Although partly an accident of history, novel chemical properties were potentially viewed as liabilities in achieving GRAS designation (Generally Regarded As Safe – a designation involving an approval process considerably less rigorous than an FDA drug approval) or end user acceptance. Since the TRPM5 modulators that we had discovered were derived from drug-like compound libraries, there was considerable pressure from Givaudan to find alternative sources of compounds to screen, preferably from natural product sources. Meanwhile, recognizing that there could potentially be other uses for our compounds beyond tastant modulation, we changed the name of the company from Linguagen to Redpoint Bio to reflect a broader focus on pharmaceutical applications.
Attitudes in the food and beverage industries were changing rapidly as the Givaudan collaboration progressed. This change was catalyzed by the introduction of the all-natural sweetener Truvia. Truvia is a low calorie sweetener developed through a collaboration between Coca-Cola and Cargill. Coca Cola had been investigating stevia glycosides as potential natural non-caloric sweeteners and had identified and obtained GRAS certification for a purified stevia leaf component rebaudioside-A (RebA). RebA initially tastes sweet but has a cloying bitter aftertaste. However, by combining RebA with erythritol, a non-metabolized and sweet poly-alcohol developed by Cargill, the bitter aftertaste of RebA can be suppressed, producing an acceptable product in Truvia. The success of Truvia excited the search for additional novel all-natural tastants and enhancers.
The desire to screen natural compounds to discover new all-natural tastants created a host of difficulties when applied to the cell-based assays we were using to identify TRPM5 modulators. Consequently Redpoint developed a totally new technology termed the MOG (Multiple Operant Gustometer) that relied on trained rats to make taste discriminations.
Although the operant taste discrimination assay (aka lickometer) had a long history in taste research, the MOG randomly presented samples from a 96-well micro-titer plate and dramatically increased throughput while producing manifestly better data than obtained using conventional methodology. MOG development was quarterbacked by Kyle Palmer who had an unusual background that encompassed both molecular pharmacology and behavioral sciences. We encountered some resistance from the beverage industry regarding the MOG approach both for its use of (very well treated) animals and because it was known the rats did not detect aspartame (a well-known synthetic sweetener) as sweet. Despite the skepticism, Redpoint was able to establish the effectiveness of rebaudioside-C (aka RebC – another stevia leaf component) as a sweetness enhancer using the MOG technology. Redpoint was able to gain GRAS certification for RebC and entered a development deal for commercialization with International Flavor and Fragrances (IFF) based on its discovery.
As a result of the paradigm shift to focus on natural products and a shift in Givaudan internal program strategy, Givaudan excercised an option to early terminate the TRPM5 program with Redpoint. Termination of the Givaudan collaboration raised several issues for the company, not the least among stockholders who had recently invested in the newly public company through a reverse merger PIPE (Private Investment in a Public Entity) transaction based on potential returns in the taste enhancement business.
At the same time that we were developing technology to find natural product tastants, we were initiating a shift in company direction to capitalize on the TRPM5 small-molecule program. This effort was responding to publications emanating from the Margolskee and other labs that, in addition to taste cells, TRPM5 had been found in gut and pancreatic islet cells, where it was inferred to participate in signal transduction chains involved in glucose-dependent insulin secretion. Consequently, it was expected that modulators of the channel could potentially act as glucose-dependent enhancers of insulin secretion, which would constitute a novel and highly valuable therapeutic for the treatment of diabetes.
We expanded our scientific team and began to evaluate both our TRPM5 enhancers and inhibitors for their efficacy in a number of cellular and animal models for insulin secretion.
We were excited to find that in both cellular assays and animal models that our TRPM5 inhibitors appeared to act as glucose-dependent insulin secretogogues. The only problem was that recently published work from the Margolskee lab describing the phenotype of a TRPM5 knockout mouse suggested that enhancers of the TRPM5 channel (used in our experiments as controls) should have secretogogue activity, not inhibitors
This apparent contradiction seriously impeded our ongoing deal activity around formation of a collaborative discovery program with a major pharma company, and ultimatel necessitated a major reduction in force to conserve cash.
In the event, it turns out that constitutive knockouts in metabolic pathway components are known to occasionally produce anomalous and unexpected phenotypes; the SUR1 KATPase that is the target of the glitazones being one relevant example.
In the event, we ultimately had to revert to out-licensing Redpoint’s research and technology assets in order to wind down the company.
On a final upbeat point, Kyle Palmer was able to evolve the MOG technology used for high-throughput rodent tastant discrimination into a totally novel operant system usable by humans. This has subsequently been developed as a key technology platform for the company Opertech Bio.